The project
About EMPIR
The European Metrology Program for Innovation and Research (EMPIR) is the main programme for European research on metrology. It coordinates research projects to address grand challenges, while supporting and developing the SI system of measurement units.
EMPIR follows on from the successful European Metrology Research Programme (EMRP), which issued its final call for projects in 2013. There is an increased focus within EMPIR on innovation activities to target the needs of industry and accelerate the uptake of research outputs.
The inclusion of capacity-building activities in EMPIR is helping to bridge the gap between countries with emerging metrology systems and those with more developed capabilities.
Need
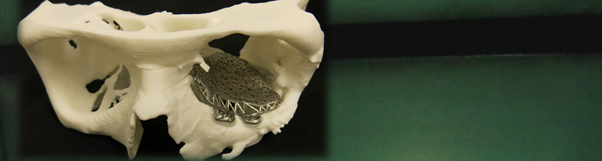
The need for this project is justified by the fact that AM technology for medical applications has advanced at a much faster pace than regulations and quality controls. Patient specific implants (PSIs) and patient specific guides (PSGs) are to be used in highly critical applications governed by strict safety requirements from notified bodies and hence controlling the quality of the parts are of paramount importance. In order for the medical device industry to have confidence in the AM technology they need validated techniques to verify the finished parts and improve the process and reliability of the manufacturing chain.
In order to validate these techniques, medical devices and standard objects, manufactured using different AM processes and materials, need to first be fabricated and characterised. Relevant aspects that have to be taken into account for these characterisations are the dimensions of external and internal geometry as well as internal defects, roughness and porosity, which will also influence the mechanical properties of the medical devices. Work is required to a) determine the precision limits of dimensional measurements and the relative sensitivity of industrial and medical XCT, and to b) qualify alternative, faster and cheaper non-destructive characterisation techniques, for routine control.
The manufacturing process of patient specific medical devices with AM contains a number of steps, from the prior CT scan of the patient to the final manufacture and clinical use, each of which can introduce errors. The material used also has an influence on the parts as well on the category of processes used. Manufacturers need tools and protocols for the detection and quantification of defects so that the best material and manufacturing process can be reliably selected. It is therefore necessary to characterise the parts at various stages in the production and application process to quantify errors in the chain from medical imaging to clinical use.
Objectives
The scientific and technical objectives of this project are:
1) To fabricate and characterise industrial medical implants, guides, and standard objects using destructive and non-destructive techniques (such as THz-CT, and XCT) and produce a good practice guide on the choice of a best suited characterisation technique. The implants, guides and standard objects will be made using different AM processes from materials such as polymers, ceramics, and metals and will be dense or lattice structures.
2) To validate non-destructive characterisation techniques, develop traceable measurement capabilities and quantify dimensional measurement errors in the whole process of personalised body part replication and standard production parts including image analysis.
3) To provide feedback to the manufacturing chain that enables process chain corrections to be implemented and manufacturing chain monitoring to be demonstrated. This will be done with the following:
- Metrology protocols that identify geometrical deviations between the numerical model and the part manufactured in the process chain;
- Correlation of the geometrical deviations to their origin to optimize the process for personal and mass produced implants and guides. For powder particles size a range of submicron (<1 µm) to 120 µm and for defects a range between 100 µm and 400 µm will be targeted.
4) To quantify the build-up of errors from each part of the whole implant and guide manufacture chain from medical imaging to clinical use.
5) To facilitate the take up of the technology and measurement infrastructure developed by the project by the measurement supply chain (accredited laboratories, instrumentation manufacturers), standards developing organizations (ISO/TC261, CEN/TC438, ISO/TC119, etc.) and end users (implant manufacturers and clinicians).

Progress beyond the state of the art
Currently AM is used mainly for prototype purposes and the technology is not mature enough to prove its reliability for certified bodies in Europe for the final implants and guides used in surgery. This work will go beyond the state of the art by developing routine characterisation techniques as well as providing feedback to the manufacturing chain via protocols to validate these implants and guides. The current state of the art for production assurance of AM parts relies on the use of a combination of conventional tactile and optical CMMs, 3D scanners, and destructive sectioning required for measurements of the internal features as well as X-ray Computed Tomography (XCT). This project will go beyond the state of the art by investigating alternative methods such as Terahertz Computed Tomography (THz-CT), Thermography and Ultrasound (US).
Impact
Impact on the metrology and scientific communities
The qualified and traceable 3D volumetric non-destructive techniques (e.g. XCT) developed in this project for dimensional measurements will enable the metrology community to characterise the geometry of complex objects manufactured using AM. Furthermore, XCT and THz-CT are new technologies in the area of metrology. Thus geometrical measurements are lacking traceability to SI and documented uncertainty assessments. The activities in the project will contribute to the work of making XCT and THz-CT measurements traceable and give valuable input to the evaluation of uncertainties thereby increasing the number of NMIs able to obtain these systems. By publishing material on traceable measurements of AM parts, the importance of metrology and measurement uncertainty will be brought to a wider scientific audience.
Impact on industrial and other user communities
This will increase the uptake of the AM technology for manufacturing on demand and customised implants. This feedback provided to the manufacturing chain in this project will give the healthcare industry the opportunity to manufacture guides with higher accuracy, which will enable accurate cutting and placement of implants and thereby reducing the operating time as well as customised accurate implants that meet the patient’s anatomy and thus reduce the recovery time after surgery.
The good practice guides developed within this project and the input to standards will provide notified bodies evidence of the improved reliability of AM. The cost of each surgical operation will be reduced as accurate guides reduce operating time and on demand accurate and customised implants will reduce the requirement for a large inventory of different sizes and sterile storage. The reduction in operating time will therefore allow more patients to be treated since operating room time is often the limiting resource.
The reduction in inventory will reduce the amount of manufacturing required, thereby having a positive impact on the environment. In addition, in some AM processes, the raw feedstock is recycled so there is no waste matter.
European companies are at the forefront of medical device development; this project will support their work and drive uptake of higher performance medical devices. Comprehensive regulations and pre-normative measurement procedures will allow this robust growth to continue, which will increase the market acceptance of all manufactured parts.
Impact on relevant standards
The business case for all stakeholders is evident as standardisation will boost the application of this technology in critical sectors such as medical devices. The FP7 SASAM project has prioritised topics for AM standardisation: materials, processes/methods and test methods. The standardisation activity is expected to enlarge the existing AM business and will help to get acceptance in existing markets (medical and aerospace). This will be a big opportunity for innovation and economic growth in the European industry, which should enhance competitiveness and will therefore be instrumental to creating jobs in Europe.
The development of standards will help the AM industry demonstrate, to other industrial sectors, that it is a mature production technology that has the expected quality assurance and can be considered for production.
Several of the project partners are already members of both the ISO/TC 261 and ASTM F42 AM committees, as well as ISO TC213 WG10 on XCT. The results from this project will be fed into either existing or new work items as appropriate.