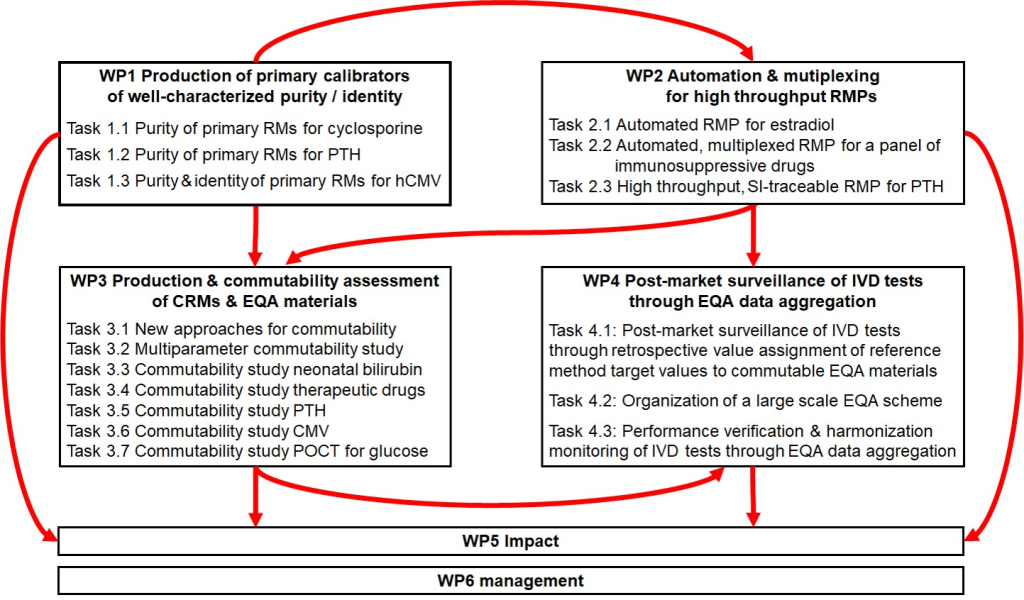



According to the in vitro diagnostic regulation (IVDR) EU/2017/746, the metrological traceability of values assigned to calibrators and/or control materials shall be assured through suitable reference measurement procedures (RMPs) and/or suitable Certified Reference Materials (CRMs) of a higher metrological order. Due to a lack of biological CRMs and RMPs (spanning from small molecules to protein to nucleic acids), IVD manufacturers are often unable to comply with regulation.
The lack of higher order RMPs to assign target values to calibrators or quality control materials is due to i) the time needed to validate high accuracy methods with fit for purpose measurement uncertainties, ii) the cost to operate such labour-intensive methods and iii) the lack of primary calibrators of well-characterised purity. There is a need for more efficient purity assessment techniques to assign target values to primary calibrators, e.g., quantitative Nuclear Magnetic Resonance (qNMR) and for high-throughput RMPs, e.g., automated LC/MS/MS methods that can be used to provide cost-effective calibration services and value assign CRMs and quality control materials.
In addition to the lack of RMPs, the lack of secondary CRMs largely originates from the difficulty and cost to properly assess materials’ commutability, which is a property describing their ability to properly mimic the behaviour of patient samples. The paramount importance of commutability has only been recognised recently and to date, however, causes for materials non-commutability remain largely unknown. As properly assessing commutability represents prohibitive costs for Reference materials (RM) producers, there is a need for more efficient and cost-effective ways of conducting commutability studies to identify suitable manufacturing processes.
To support manufacturers in post-market surveillance, there is a need to improve availability of External Quality Assessment (EQA) schemes using control materials of proven commutability and reference method target values.
Addressing these needs requires a coordinated metrological response involving all stakeholders: IVD manufacturers, EQA providers, RM producers, international organisations promoting standardisation.
The overall aim of this project is to establish the necessary metrological infrastructure to provide calibration services to External Quality Assessment (EQA) providers and IVD manufacturers to evaluate and improve accuracy and harmonisation of medical test results. This will help the in vitro diagnostic industry meet the requirements of the In Vitro Diagnostic Medical Devices Regulation (IVDR) regarding calibration and performance verification of IVD tests. The specific objectives are: