
A LNE Joint Research Project


One out of eight people in Europe is affected by diseases of the nervous system (Alzheimer‘s and Parkinson’s disease, multiple sclerosis, epilepsy, brain tumours, paraplegia etc) with costs associated to this group of diseases corresponding to one third of the healthcare expense in Europe before the pandemic. Early and accurate diagnosis of NDDs is crucial for effective management of diseases and early identification of patients who could most effectively benefit from new treatments. The number of fluid biomarkers available for early diagnosis of neurodegenerative diseases is growing together with the rapid development of assays that have the potential to be routinely used in clinic. Translational research and metrology have a key role to play in this landscape.
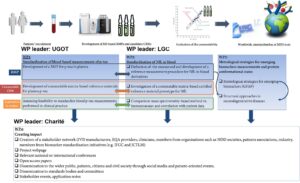
While research in NDDs has significantly progressed in the last few years through the discovery of new biomarkers and the development of non-invasive sensitive assays implemented on automated platforms, large-scale implementation of these biomarkers in routine clinical settings necessitates standardisation. To date, most initiatives striving for standardisation of NDD biomarkers are focused measurements performed in cerbrospinal fluid (CSF), including Amyloid Beta 1-40 (Aβ 1-40), Amyloid Beta 1-42 (Aβ 1-42), total tau (t-tau) and Neuro Filament Light chain (NfL). To meet the need for minimally invasive assays, there is an urgent need to standardise measurements of NDD biomarkers in blood. Building a European infrastructure for NDD biomarker standardisation is of utmost importance to rapidly implement new NDD biomarkers in clinical practice. Due to the structural heterogeneity of these biomarkers, detailed molecular characterisation of the analytes requires metrology to define the measurand and develop adequate reference method procedures (RMPs) and certified reference materials (CRMs).
This project will develop a metrological research framework for standardising biomarkers of neurodegenerative diseases (NDDs), including Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia and Parkinson’s disease, the fast implementation and commercialisation of promising assays for NDD biomarkers will be promoted through compliance to the EU IVDR 2017/746. The specific objectives are: