


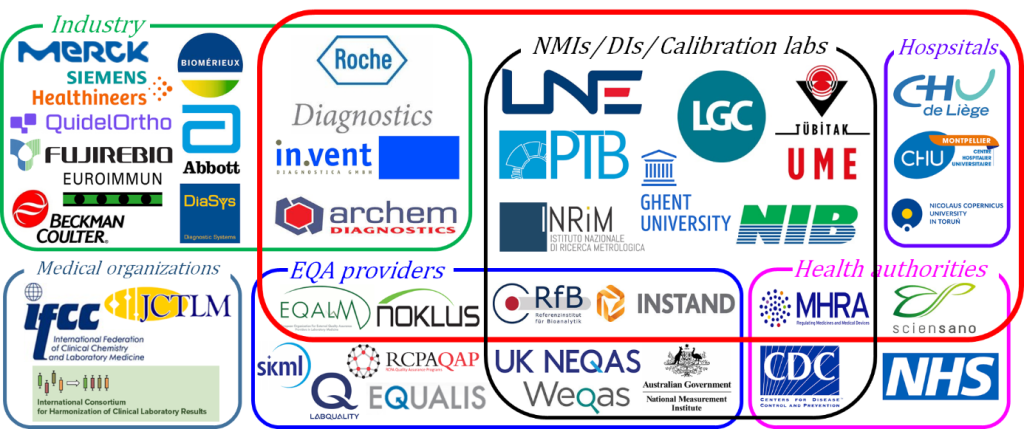
The consortium is an ideal network to develop new measurement capabilities and reference measurement systems for clinically relevant measurands. Participants of the consortium have a long history of collaboration through National, European, and international projects, including EMRP / EMPIR projects, IFCC and JCTLM Working Groups, ISO and CLSI committees. The Consortium gathers participants that can be classified in 4 groups that will strongly interact with each other:
LNE has extensive expertise in developing reference methods and reference materials for clinically relevant biomarkers. As chair of the European Metrology Network TraceLabMed, LNE is in an ideal position to coordinate the project. In addition, LNE will also support the other participants in various activities of all other WPs. Thanks to its expertise in commutability, LNE will lead the design of new approaches for commutability assessment in Task 3.1 and the organisation of commutability studies. In WP1, LNE will contribute to the purity assessment of primary calibrators in Tasks 1.1 and 1.2. Having established an extensive network of key opinion leaders, clinicians, health authorities, regulators, reference laboratories, assay manufacturers and industry participants, LNE will lead the Impact WP (WP5). Having successfully coordinated a number of multi-participant projects, LNE has the infrastructure and experience needed to successfully manage this project and will also coordinate WP6. In the healthcare sector, LNE currently coordinates EPM projects NEuroBioStand (22HLT07) and ProMET(23FUN06).
INRIM is active in the Nucleic-acid Analysis Working Groups of the CCQM. INRIM is actively working to provide primary measurement capabilities and to generate SI-traceable reference values for nucleic acids measurands by digital PCR. INRIM main contribution will deal with hCMV-related activities, i.e. Task 1.3 and Task 3.6.
NIB brings strong expertise in nucleic acid analysis with molecular methods such as qPCR, dPCR, isothermal amplification, sequencing and their development and standardisation. NIB is also experienced in extraction of nucleic acids from different matrices. It is accredited to ISO 17025 and operates a reference method for hCMV listed in JCTLM database. NIB main contribution will deal with hCMV-related activities, in Tasks 1.3 and 3.6.
PTB provides primary measurement capabilities as national standards for important measurands in clinical chemistry (electrolytes, metabolites, proteins, cells, and nucleic acids) and generates SI-traceable reference values for these measurands in EQA and interlaboratory comparisons for reference laboratories (e.g., IFCC RELA surveys). PTB has extensive expertise in purity assessment of biomolecules and will lead WP1.
TUBITAK is the National Metrology Institute of Turkey. Having a strong expertise in purity analysis of various types of measurands (metabolites, proteins, and nucleic acids), TUBITAK will have an important contribution in WP1. As a reference materials producer with significant production facilities, TUBITAK will also contribute in WP3 through materials production and value assignment of reference method target values.
CHUL is the University Hospital of Liège (CHU de Liège), Belgium. CHUL chairs the IFCC Committee on Bone markers (IFCC C-BM), in which PTH standardisation is a priority. CHUL clinical laboratory is equipped with almost all major clinical analysers for immunoassays and operates a RMP for PTH. These will be used to analyse samples of the PTH commutability study in Task 3.5, in which CHUL will recruit all patient samples.
CHUM is a French University Hospital which combines complementary activities in clinical diagnostics. The first is clinical biology lab (ISO 15189) implementing, validating and developing routine and research assays for clinically relevant biomarkers. The second is biological resources (NFS 96-900) for collections which regroups more than 18000 biological samples. CHUM will be the lead participant in charge of recruiting clinical specimens for commutability studies conducted in WP3. CHUM will also provide general clinical expertise.
HDS is the Norwegian Organisation for Quality Improvement of Laboratory Examinations and is a national evidence based non-profit organisation. HDS is a major contributor of the IFCC WG on commutability and will lead WP3. HDS will contribute to the development of new statistical approaches for accelerated commutability assessment. HDS has world-leading expertise in POCT and will lead Task 3.7.
INSTAND is a German non-profit scientific medical society acting as an EQA provider accredited according to ISO 17043. It operates its own calibration laboratory which is accredited according to ISO 17025 and ISO 15195. The main contribution of INSTAND will be on WP3 on commutability with the assignment of reference method target values to EQA materials and clinical specimens. INSTAND will also provide an important contribution in POCT for glucose and contribute in EQA data evaluation in WP3 and WP4 and assignment of reference method target values to EQA materials & clinical specimens.
Sciensano is the Belgian institute for health. Sciensano performs public and animal health assignments and is recognised as a research institution by the Belgian Science Policy. Sciensano will lead WP4 on EQA data aggregation and provide general support in statistics (e.g., sample size estimation) and data analysis in WP3.
SPMD‑RfB is a German EQA provider accredited according to ISO 17043. It runs two calibration laboratories accredited according to ISO 17025 and ISO 15195 which provide reference measurement services for a wide range of clinically relevant biomarkers. SPMD‑RfB will contribute on WP3 on commutability with the assignment of reference method target values to EQA materials and clinical specimens. SPMD‑RfB main contribution will be in WP4 with the organisation of a large scale EQA scheme, assignment of reference method target values to EQA materials & clinical specimens.
UGent is the Ghent University, in Belgium. UGent is a well-established calibration laboratory, especially for steroid hormones. UGent main contribution will be the value assignment of reference method target values to EQA materials and clinical specimens in WP3 and WP4.
UMK is the Nicolaus Copernicus University in Toruń, Poland. A UMK representative chairs the JCTLM Task Force on Reference Measurement System Implementation and has world-leading expertise in the definition of analytical performance specifications and measurement uncertainty evaluation, which will be UMK main contribution in the project. UMK will also contribute to designing commutability studies and the recruitment of clinical specimens against which commutability of CRMs and EQA materials will be evaluated in WP3 and WP4.
Archem is a well-established manufacturer of IVD reagents manufacturing and marketing. With 20 years of experience for manufacturing biochemistry reagents, Archem main contribution will be in WP3 through the production of RMs and EQA materials according to various manufacturing processes to be compared.
INVENT is in.vent Diagnostica GmbH, a European leader in the targeted provision of human bio-materials use throughout the whole value chain of in-vitro diagnostics. INVENT main contribution will be in WP3 through the production of CRMs and EQA materials according to various manufacturing processes to be compared.
Roche is the Instrumental Analytics Department of Roche Diagnostics. Roche’s main contribution will be to develop new approaches for purity assessment of primary calibrators for proteins in WP1. Roche will also contribute in WP2 through the development of approaches for automation and multiplexing of RMPs.
LGC is the UK’s National Measurement Laboratory and Designated Institute for chemical and bioanalysis. LGC has outstanding experience in developing clinical CRM & higher order reference methods for biomarkers. Having a long-standing experience in automation, LGC will lead WP2. Having expertise in purity analysis of various types of measurands (metabolites, proteins, and nucleic acids), LGC will be an important contributor of WP1. LGC will also contribute to the assignment of reference method target values in WP3 and WP4. LGC will be associated to all funded beneficiaries.
MHRA is the Medicines and Healthcare products Regulatory Agency. Within UK’s Department of Health is the National Institute for Biological Standards and Control (NIBSC), a UK Designated Institute with recognition for bioactivity metrology. MHRA expertise in the formulation, freeze-drying, and subsequent value-assignment of RMs for complex biologicals will be applied to the production of new WHO International Standards for PTH and hCMV which purity will be evaluated in WP1, and commutability evaluated in WP3. MHRA will be associated to all funded beneficiaries.